Clouds are beautiful! You look up to the sky and you see them in different shapes and sizes. What’s even more amazing is that they have different names for the different shapes; like Cirrus, Nimbostratus, and Cumulus. Though, the biggest question we had when we all saw those beautiful clouds (or sometimes sad clouds) is how are they made?
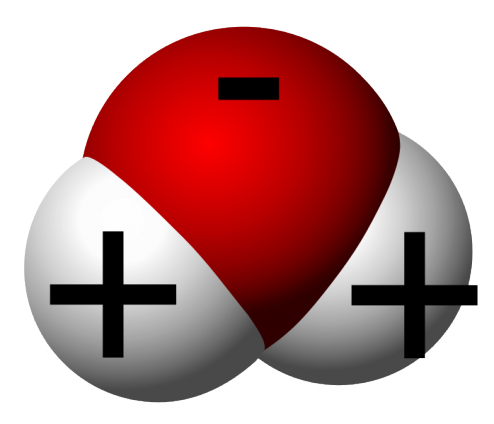
A cloud is a large collection of water droplets, very tiny ones, or ice crystals. Since the droplets are super small and tiny, they can float up in the air.
Actually, all air contains water to be practice. However, when we are near the ground level, or sea level, water is usually in the form of water vapor, which is invisible to us because of the distribution of water molecule in the space provided (imagine a pea in a football stadium and you are seeing the stadium from a helicopter).
When warm air rises, it expands and cools due to changes in the atmosphere itself. When the water vapor gets cold, the water molecules come together slightly closer and form tiny droplets; just like how we cuddle with someone when we get cold ourselves. This process is called condensation, when air molecules condense due to cold and slowly form into a liquid. When billions of these droplets come together, yet they are spaced not too far from each other, they become a visible cloud.
How does condensation work?
Condensation forms when water changes from a vapor (or gas) to a liquid. Consider the water molecules as people. For people to go around, hang out and meet with friends, they need energy. In the presence of heat, there is energy for things to happen (not extreme heat, of course).

People cannot stand extreme heat; otherwise, this place would have been packed!
So water molecules party it up and rock out everywhere in the air because of decent heat. However, as the water is cooled and there is less heat energy for the individual molecules and particles to move around, it then condenses. Just like cuddling. Therefore, condensation happens because of change in temperature.
How do clouds float?
Clouds are heavy. The water in a cloud can have a mass of several million tons. So, how do they float?
 Cloud droplets are also about 1000 times heavier than evaporated (gas) water, so they are much heavier than air. However, they do not fall, but stay in the air, because there is warm air all round the heavier water droplets. You can imagine the warm air being bullies pushing the water to be together into a droplet.
Cloud droplets are also about 1000 times heavier than evaporated (gas) water, so they are much heavier than air. However, they do not fall, but stay in the air, because there is warm air all round the heavier water droplets. You can imagine the warm air being bullies pushing the water to be together into a droplet.
When water changes from gas to droplets, this makes heat. Because the droplets are very small, they “stick” to the warm air. And thus, they float.
The Experiment
The Summary
Make some clouds in a bottle.
What you need:
- Tea spoon
- A 2-Liter bottle and a cap
- Matches/Fire
- Piece of Newspaper
Steps:
What’s going on?
As the video mentioned, the increase in pressure and decrease in pressure is increasing the temperature (remember how hot it is when using a bicycle pump?). This phenomena is better explained by this glorious formula, ideal Gas Law:
PV = nRT

No, we don't measure gas with those moles. Nor the moles on your body!
Where the volume (V) is constant because your gas is in the bottle of volume 2 liters, and there is a constant amount of gas (n) measured in moles, R is the ideal, or universal, gas constant, equal to the product of Boltzmann’s constant and Avogadro’s constant. And finally, T is temperature. As pressure changes often to higher pressure on average, it increases the change in temperature, leading to higher temperature (since the original temperature is constant).
Given the increase in temperature, change in pressure, and presence of water molecules in the bottle, clouds are formed satisfying the conditions and explanations mentioned above!