
The Titanic can be considered a Tension Boat
The title may be misleading; one might think it is a very painful cruise ship vacation experience. However, today’s experiment will focus on surface tension of water and how we can use this property to propel objects on water. Surface tension can be seen everywhere in nature. Some examples include:
- Water beads on waxy sufaces, like tree leafs or windows that are super clean. Water adheres weakly to wax and strongly to itself. Which leads to the phenomena of water drops coming together and forming a large drop on these surfaces
- Separation of water and oil is caused by a tension in the surface between not-similar (or dissimilar) liquids. Normally, this is called Interface Physics, but its the same thing
A tension boat is basically a boat that utilizes the property of surface tension of water to propel itself in water. You might think that you can gain infinite propulsion force by placing ships in the ocean with enough material to harness this force and have green energy. But, it’s much more difficult than that, and I don’t think carrying gallons of soap and ocean water would work that easily.
What is Surface Tension
The straight up definition: The cohesive, or attractive, forces between liquid molecules causes surface tension.
Let’s consider the following image of a water molecule:
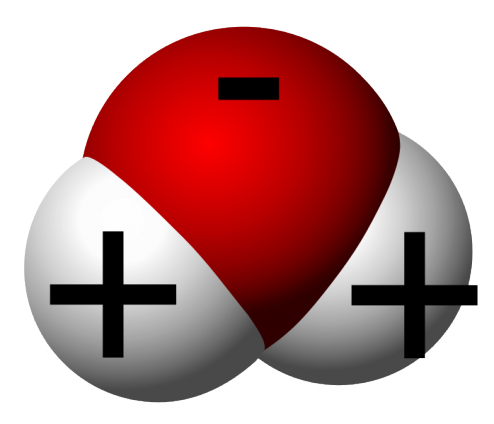
MEGA MASSIVE Water Molecule
As you can see, the water molecule consists of three atoms; two Hydrogen atoms (represented as white and positive) and an Oxygen atom (represented as red and negative). The “charges” you see on the molecules are due to the bonding between the Oxygen and Hydrogen atoms.
When Hydrogen gives away (shares out) their electron, it becomes positive (it hates being negative; imagine Hydrogen as someone high on life). On the other hand, Oxygen is like negative nancy or debbie downer, always negative and LOVES to get (or borrow) electrons.
Based on this property, it creates a “polar” molecule, which basically means that the molecule is like a magnet. A super super super super super super super super small magnet. And like any other magnets we nearly swallowed as a child in the world, they attract each other. Negatives attract the positives, and vice versa, and then you get like a chain of water molecules together. Therefore, in a glass of water, you have a party going on with the water molecules!
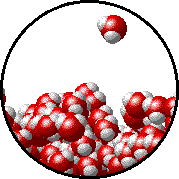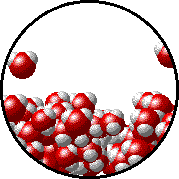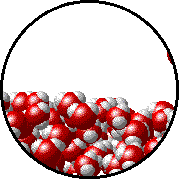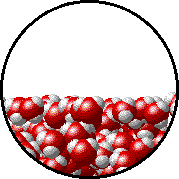
Water Molecules Partying to LMFAO!
As you can see in the party, the water molecules in the glass (or middle of the glass) are attracted from all sides and are influenced or “pushed” by other water molecules. However, for the water molecules on the surface, since they are not attracted from all sides (their top being open to air), they form a stronger attraction with their water molecule buddies around them. Because of this stronger attraction to the molecules to the sides of the water molecule, the surface of the water acts like an elastic surface!
And that, ladies and gentlemen, is how we have suface tension!
The Experiment
Summary
Make a small boat out of cardboard, place in water, add a drop of soap at the back-end of the boat, and watch that thing go!
What you need:
- Water
- Cardboard box
- Soap or Detergent
- Scissors
- A clean kitchen sink, or round cake pan
Steps
- Cut the cardboard with scissors in one of the following shapes (You can make your own boat, just notice the back-end of the boat having an internal dent)
- Place the cardboard slowly on the surface of water (whether in the sink or round cake pan)
Sing a Pirate Song!- Add a drop or two of soap on the backend of the boat. Slightly dab the soap on the back
- See that bugger go!
What’s Going On?
So, you know about surface tension at this stage, and you noticed before you dabbed the boat with soap that the boat was still (unless your sink or whatever you used is so dirty and your boat went berserk, you dirty person you!).
The reason why the boat is still when placed in water is because the surface tension on the surface of water is acting on “all-directions” on the boat.
Let’s examine the property of soap. Soaps are compounds which are made by heating fats or oils, from animal or vegetable sources, with lye. A typical soap molecule has the formula:
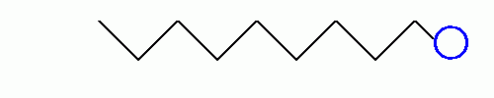
Looks like an electrocuted sperm...
Soap belongs to a class of chemicals known as surfactants, from surface active agents. As yours parents said, soap have some special properties which make them very useful for cleaning and forming bubbles and foam. In particular, the long hydrocarbon (All that C’s and H’s on top) ends of the molecules are very nonpolar and do not form bonds to water molecules. This end is hydrophobic (water fearing). On the other hand, the ends are very soluble in water and form rather strong bonds with the very polar water molecules. Those are hydrophilic (water lovin’).
When soap is placed at the end of the boat, the soap molecules order themselves in the position that will distort the surface tension of the water. The water molecules on the surface of the pool of water will lose their adhesiveness to each other because of the hydrophilic part of the soap. On the other end, water molecules are distancing themselves from the tail of the soap molecule. This can be shown in the following graph:
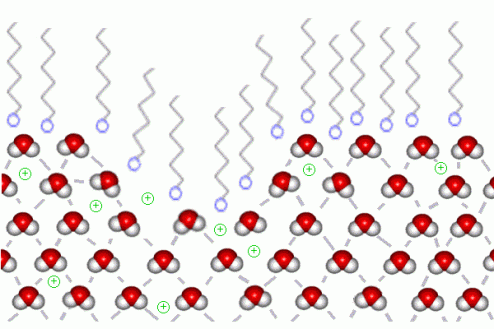
Soap molecules hatin' on water
Based on such interaction, aggregate effect of all forces will be “frontward” rather than “all-direction.” Such effect creates propulsion. Thus, you are capable of making the most awesome soap propelled speed boat!
Sources:
- California State University Stanislaus – Chemistry Department
- How Soap Floats Your Boat?
- Wikipedia – Surface Tension
- George State University – Physics Department

